Softening units:
Function and advantages
Hard water often leaves lime residue. To avoid these traces from the outset, softening plants, colloquially also called decalcification plants, are used. But how do they work, what advantages do they offer and how exactly is lime actually produced? We can give you the answers:
Traces of lime are everywhere – from the sink and dishes to the kettle and coffee machine. But limescale deposits are also noticeable in heating and pipe systems – at the latest when the pipes are closed and the water pressure decreases. With decalcification systems, such damage can be reduced to a minimum in advance. In addition, soft water can also be used to significantly improve the aroma – for example when preparing coffee or tea.
What is lime and how is it formed?
Lime consists of dissolved calcium and magnesium compounds. It is a natural component that occurs in many layers of rock and soil. Through dissolution processes, it reaches ground and surface water. A large part of the local water supply is covered by groundwater. In this way, the mineral-enriched, calcareous water reaches households via the respective water supplier.
The minerals contained in water, calcium and magnesium, are also known as hardness builders. They are harmless to human health and even vital. For example, they promote the development of teeth and bones. The daily requirement of a person is sufficiently covered by food. However, an increased content of these hardness builders in water can lead to stubborn deposits and encrustations on fittings and household appliances – for example on the heating rods of your kettle. If the water is heated or there is a drop in pressure – such as when it leaves the single-lever mixer – the calcium and magnesium compounds dissolved in the water precipitate and a white deposit – commonly known as lime – forms.
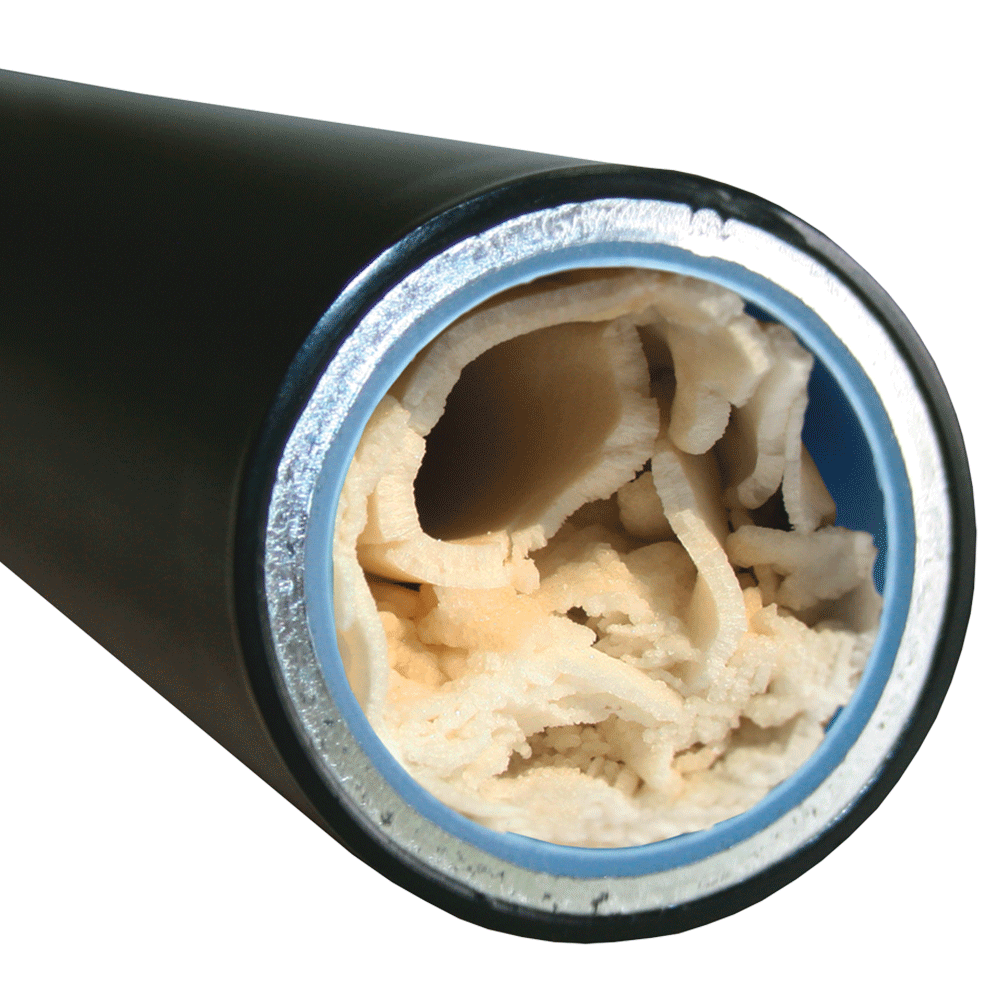
Deposits lead to functional damage and increased energy consumption
Even low degrees of hardness can cause deposits in the kitchen, bathroom and the pipe and heating system when heated. This not only increases the amount of cleaning required, but also leads to higher energy consumption and thus to growing costs. A lime layer of one millimeter corresponds to an increase in energy consumption of about ten percent. Another negative effect is that lime deposits, due to their surface structure, favour the colonisation of bacteria.

The ion exchange principle of descaling units
Softening units are suitable for stopping lime precipitation before it forms. In contrast to conventional simple table filters, they soften the water before it enters the pipes and thus have a double effect – in the installation as well as in the preparation of food and personal hygiene. The soft water leaves virtually no lime residue in the pipes, on fittings or household appliances and also has a positive effect on taste and well-being – for example when bathing or showering. Softening systems such as those in the i-soft series from JUDO work on the principle of ion exchange. Calcium and magnesium in the water are filtered out by resin beads and replaced by sodium. The exchange causes the lime-forming substances to be removed. Decalcified water is also called soft water.
The water hardness of the DVGW-tested i-soft systems can be adjusted to suit your needs. They compensate for fluctuations in water hardness fully automatically and adjust the degree of hardness to suit the situation. The operator can easily control the system via app or web browser. This means a significant plus in comfort. The advantages in the household: With softened water, you can save up to 50 % detergent. In the ideal case, you can even do without fabric softener or rinse aid altogether. The consumption of products such as shampoo, hair conditioner or soap is also reduced. In addition, soft water improves the skin texture and makes hair easier to style. This means you can significantly reduce your expenditure on cosmetics, rinsing agents and detergents.
An overview of possible advantages of softened water:
- Hardly any lime residue on fittings and appliances
- Deposits in pipes and heating systems are reduced to a minimum
- Optimized water pressure and flow rate
- No increased energy consumption due to deposits in the water heaters or in heating systems
- Reduced risk of colonisation of the domestic installation with germs
- Saving of detergent by up to 50 percent
- Higher life expectancy for technical appliances such as washing machines, dishwashers, kettles or high-quality espresso machines
- Significant savings on fabric softener and rinse aid
- Can improve skin texture
- Sustainable savings in hair and skin care products
Besides using a softening unit, the hardness in the water can also be treated by adding mineral solutions. JUDO offers special dosing pumps for this purpose. The i-dos dosing pump, in combination with the appropriate mineral solution from JUDO, stabilises the lime in the water. The pump monitors, controls and doses the water independently. The dosing systems and our mineral solutions meet all requirements of the german drinking water ordinance and are DVGW-tested. This means you always receive the best possible water quality. In addition, i-dos also independently provides the prescribed documentation of the treatment substances on demand.
The water cycle
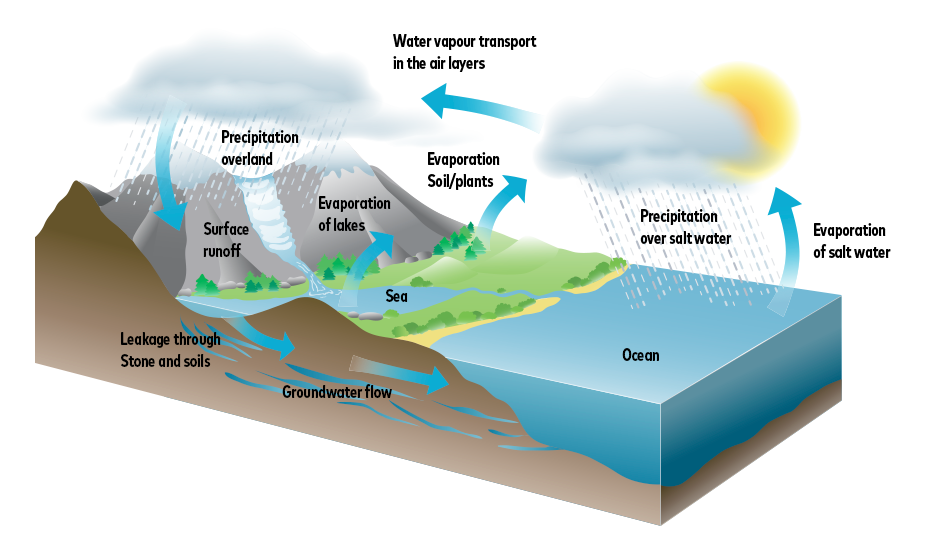
The water evaporates from seas, rivers or lakes and rises with the warm air. At altitude, the air cools down again, the water vapour condenses and forms clouds. The water returns to earth in the form of rain, snow or hail.
Rainwater already absorbs various substances in the atmosphere, which greatly change its properties (carbon dioxide, sulphur dioxide, dust, etc.) Carbon dioxide (CO₂) is of particular importance here, as it influences the solubility of the water, especially for the lime formers. This is a gas that can be produced during the burning of coal or oil as well as during respiration.
As it flows through the soil layers, water accumulates ever greater quantities of substances. In addition, microorganisms are always present, and legionella is also found in practically all naturally occurring waters.
The occurrence and composition of the minerals contained in the soil determine the hardness of the water: If a large amount of calcium and magnesium ions get into the water, it becomes hard. This is particularly the case in areas with a lot of limestone or chalk-containing rock.
 © JUDO 2024 | All rights reserved.
© JUDO 2024 | All rights reserved.